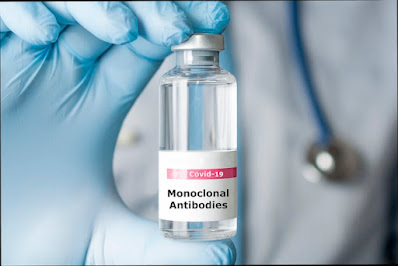
The Medicines and Healthcare products Regulatory Agency (MHRA) has given approval for the first monoclonal antibody treatment for the prevention and treatment of COVID-19 in the UK.
Following on from a thorough review of the evidence carried out by the MHRA, and recommendation by the Commission on Human Medicines (CHM), the government’s independent expert scientific advisory body, the MHRA has approved Ronapreve as the first monoclonal antibody combination product indicated for use in the prevention and treatment of acute COVID-19 infection for the UK.
Developed by Regeneron/Roche, the drug is administered either by injection or infusion and acts at the lining of the respiratory system where it binds tightly to the coronavirus and prevents it from gaining access to the cells of the respiratory system. Clinical trial data assessed by a dedicated team of MHRA scientists and clinicians has shown that Ronapreve may be used to prevent infection, promote resolution of symptoms of acute COVID-19 infection and can reduce the likelihood of being admitted to hospital due to COVID-19.
Source: www.gov.uk



No comments:
Post a Comment